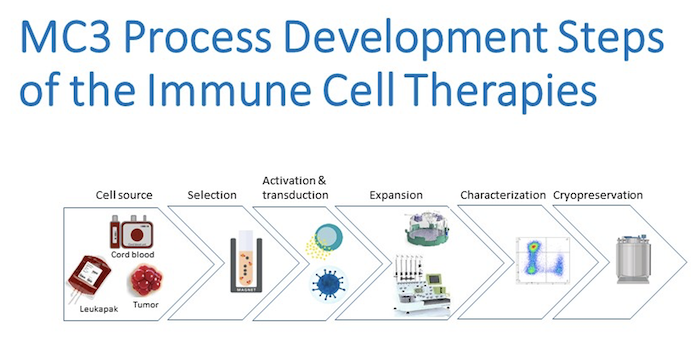
Detailed manufacturing protocols need to be developed to translate cell or biological therapies from the research bench to bedside. The Process Development (PD) Laboratory at the Marcus Center for Cellular Cures will convert your protocols derived from pre-clinical studies into GMP (current Good Manufacturing Process)-aligned manufacturing protocols to achieve high-quality and effective clinical products for use in clinical trials. The process development goals are to define cell manipulation steps, simplify the process, implement GMP grade reagents and components, and reduce production cost. We also establish the assays for release testing and develop potency assays to assess the efficacy of the final product.
Located in downtown Durham in the historical Chesterfield building, the PD team has a spacious and well-equipped lab. We have several biological safety cabinets, cell culture incubators, automated cell counters, flow cytometer analyzers, bioreactors, controlled rate freezers, and other cell processing devices. We have years of experience in generating and expanding cells from various cell sources, such as peripheral blood, cord blood, umbilical cord tissue and tumor tissues for both autologous and allogeneic therapies. The cell production is scalable from small-scale flask systems to larger bioreactors.
Completed manufacturing and release testing protocols are transferred to the GMP and Quality Control teams, respectively. The transfer includes staff training and Standard Operating Procedure (SOP) and batch record writing. The PD team works closely with the research lab, CCBB (Carolinas Cord Blood Bank), GMP, quality control and assurance and regulatory units at MC3 and collaborates with principle investigators internally at Duke and externally.
Tasks performed at the PD Laboratory:
- Cell product protocol development
- Protocol optimizations and troubleshooting
- Reagent replacements
- Release assay development
- Potency assay development
- Device testing
- Standard operating procedure and batch record writing in collaboration with the GMP team
- Formal studies for amendments of FDA-approved protocols
The PD Laboratory has been focused on the development of immune cell therapies (Tumor infiltrating lymphocytes, NK cell, CAR-T cells).